
The experiment consists of studying extremely small drops of oil that are located between two horizontal parallel metal plates. The plates may be connected to a potential to produce an electric field which will affect the drops that are charged. If the electric field is turned off the drops will fall subject to two forces: the force of gravity mg and a force due to the frictional effect of viscosity. This force acts opposite the direction of motion and is given by Stokes law:

where 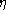 is the viscosity, r the radius of the drop and v' its speed.
The speed will quickly increase until F_s = mg after which the
terminal velocity v' will remain constant. If we replace m by the
product of the density of the oil
is the viscosity, r the radius of the drop and v' its speed.
The speed will quickly increase until F_s = mg after which the
terminal velocity v' will remain constant. If we replace m by the
product of the density of the oil 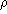 and the volume of the drop, the
equilibrium condition is
and the volume of the drop, the
equilibrium condition is

which may be solved for r to give:

The electric field is now turned on and its polarity adjusted so that the drop moves up. The force up is now qE and the force down is mg + F_s. Again a constant terminal velocity is quickly reached such that

If we again use

and the above expression for r, this gives:
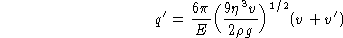
This would be the final expression except for a small correction,
first noted in fact by Millikan during the course of this experiment.
It turns out that Stoke's law must be modified when one considers
the viscous retarding force on very small spheres. The effect is
to divide the viscosity by the factor (1 + b/pr) where b is a
constant and p is the pressure. In the above equation for r, if
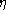 is replaced by
is replaced by 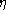 /(1 + b/pr)
and the resulting quadratic equation
solved for r, the result is:
/(1 + b/pr)
and the resulting quadratic equation
solved for r, the result is:
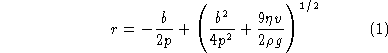
In the above equation for q', if 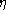 is replaced by
is replaced by
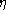 /(1 + b/pr)
and Eq. (1) is used for r, the result can be simplified to give:
/(1 + b/pr)
and Eq. (1) is used for r, the result can be simplified to give:
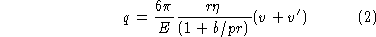
These two equations, which contain only constants and directly measured quantities, are to be used to calculate q.
The toggle switch incorporated as part of the apparatus is a reversing switch with a center position in which the plates are shorted, to insure zero field between the plates while the free- fall velocities are being measured. Connect the two plates to the binding posts on this switch marked "Condensor". Connect the high voltage supply and a voltmeter to the binding posts marked "High Voltage Input".
CAUTION The plates to which the high voltage is connected are exposed and can give quite a jolt to the person working on the apparatus with the toggle switch in other than the center position.
Careful adjustments of the microscope and light are essential if the drops are to appear as bright dots on a black background. This must be done by trial and error, but generally the light beam should be slightly converging and collimated enough that it does not strike any surface inside the chamber. The microscope should be initially focused on a pin inserted through a small hole in the top of the chamber.
Spray some oil into the cylinder placed over the holes in the top plate. Some oil will drift through the holes and should be visible rather soon after the oil is atomized into the cylinder. The drops will appear to rise since the telescope produces an inverted image. Select a drop that takes several seconds to drift between the divi- sions on the microscope reticle. Find the position of the switch that will cause the drop to change direction when voltage is applied. The velocity in the reverse direction should also be small. Toggle the switch so that the chosen drop changes directions a few times before starting to take data. This should remove most of the drops in the vicinity of the chosen drop in order to effectively isolate it. It is suggested that one person observe the drop, start and stop the timer and manipulate the toggle switch while someone else records the timer readings and resets it. Take many readings of rise and fall times using the same drop. If the velocity suddenly changes during a rise (apparent fall) it is because the charge on the drop has changed. Note the change and disregard that particular rise time, but continue taking readings on the same drop. If after ten or so readings the charge has not changed, try to induce a change by placing a radium-E needle close to the holes in the top plate. Try to observe several charge states with the same drop. If the drop is lost, continue with another drop. If the drop drifts out of focus, move the entire microscope to bring it back into focus. Let each member of the group take a turn observing the drops.
Calibrate the microscope by observing a scale placed in the focal plane. Take observations of the other parameters needed to complete the calculation: atmospheric pressure, temperature, high voltage and spacing between the plates. Other constants and parameters needed in the calculation are:
b = 8.13e-8 m-atm
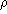 = [894 - 0.67(T - 20°C)] kg/m³3
= [894 - 0.67(T - 20°C)] kg/m³3
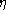 = [1.832 - 0.0049(23°C - T)]*1.0e-5 joule-sec/m³3
= [1.832 - 0.0049(23°C - T)]*1.0e-5 joule-sec/m³3
Use the average of all fall times for a given drop and the micro- scope calibration to calculate the average free-fall velocity v. The rise times should fall into obvious groups. Use the average rise time for each group to calculate an average rise velocity v' for that group. Calculate the corresponding value of q using Eqs. (1) and (2). Express the result as an integral multiple of a number close to e, the electronic charge. Compute the mean value of e from all your results and the RMS deviation.
Discuss the extent to which your results show that charges exist as integral multiples of a fundamental quantum of charge. Is your mean value of e within your RMS deviation of the accepted value? If not, discuss possible reasons. Discuss any spurious results.
© Department of Physics and Astronomy, University of Iowa, Iowa City, Iowa. All rights reserved.