29:30 Physics IV Experiment 11
Experiment 11 Atomic Spectra
Purpose:
- To observe atomic line spectra from several discharge tube
sources.
- To measure wavelengths of prominent lines in these spectra with
precisions of 0.1% or better
- To verify parts of the term diagrams pertaining to the observed
lines.
Discussion:
The data which provided the main impetus for the development
of quantum mechanics, as well as some of the most important tests
of the theory, were atomic spectra. Large devices and long exposure
times are necessary for the most precise data. Spectrometers with
film strips 30 feet from a diffraction grating and exposure times
of several hours are not uncommon. However, quite respectable data
can be obtained with a small laboratory spectrometer if care is
taken. In this experiment you will use a constant-deviation prism
spectrometer to observe spectra from several different sources.
The spectrometer gets its name because the prism is ground so that
the angle between the incident light beam and the observed beam
remains at 90° as the prism is rotated by a dial calibrated in
Angstrom units.
Procedure:
Observe the Hydrogen spectrum.
The visible lines are in the
Balmer series. Measure as many as you can (four should be observable)
and obtain a preliminary set of values of
 (
(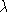 )
versus
)
versus 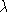 where
where  (
(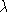 ) is
the difference between the dial reading and the known wavelengths
of the observed lines. The idea here is that the spectrum is very
simple so there should be no ambiguities, and the wavelengths are
known exactly from a theoretical expression. Take at least five
measurements of each line and use the average for your graph. Notice
there is considerable backlash in the dial so it will be necessary
to always approach the final setting from the same direction.
) is
the difference between the dial reading and the known wavelengths
of the observed lines. The idea here is that the spectrum is very
simple so there should be no ambiguities, and the wavelengths are
known exactly from a theoretical expression. Take at least five
measurements of each line and use the average for your graph. Notice
there is considerable backlash in the dial so it will be necessary
to always approach the final setting from the same direction.
Observe the spectra of mercury vapor and sodium vapor.
The lines in
the mercury spectrum are commonly used as spectrometer calibration
points because of their isolation, prominence and spread. Take five
or more readings of each line and find the averages. Use your
preliminary calibration curve if necessary to positively identify the
lines and construct an improved calibration curve. Include this
curve in your report and use it to correct all future measurements.
The bright yellow lines in the sodium spectrum are the famous D-lines.
Find their wavelength and estimate their spacing. Find the wavelengths
of other lines in the sodium spectrum and observe that they
are all doublets. Estimate these doublet spacings wherever possible.
Observe the helium spectrum.
It should be possible to obtain wavelengths of from 10 to 15 lines.
Observe spectra from other gaseous discharge tubes available.
Determine the wavelengths of the prominent lines and count the number
of weaker lines between the stronger ones. Note sufficient comments
to allow completion of column E in the table mentioned below.
Report:
- Prepare a table summarizing all your observations as follows:
Column A, dial reading; Column B, corrected wavelength; Column C,
accepted wavelength; Column D, error; Column E, relative intensity.
For column E use the following standard notation: VS, S, M, W or VW
for Very Strong, Strong, Moderate, Weak or Very Weak, respectively.
- Discuss the accuracy of your measurements. That is, answer the
following question: Based on your experience in measuring numerous
spectral lines, what is the uncertainty in the corrected measured
value of any given measured wavelength?
- Discuss the various techniques you may have developed. (E.g.,
concerning backlash, slit width, source positioning, focus, etc. How
are these things dealt with and what is their effect on the accuracy
of the results.)
- Find the term diagram for helium in some textbook or other reference
and reproduce it in your report. Show with heavy lines the transitions
that give the lines you have observed.
© Department of Physics and Astronomy, University of Iowa,
Iowa City, Iowa. All rights reserved.
 (
(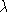 )
versus
)
versus 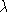 where
where  (
(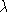 ) is
the difference between the dial reading and the known wavelengths
of the observed lines. The idea here is that the spectrum is very
simple so there should be no ambiguities, and the wavelengths are
known exactly from a theoretical expression. Take at least five
measurements of each line and use the average for your graph. Notice
there is considerable backlash in the dial so it will be necessary
to always approach the final setting from the same direction.
) is
the difference between the dial reading and the known wavelengths
of the observed lines. The idea here is that the spectrum is very
simple so there should be no ambiguities, and the wavelengths are
known exactly from a theoretical expression. Take at least five
measurements of each line and use the average for your graph. Notice
there is considerable backlash in the dial so it will be necessary
to always approach the final setting from the same direction.