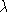 = h/p (1)
= h/p (1)
In this experiment you will verify the DeBroglie relation
= h/p (1)
where 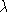 is the wave length of the particles, electrons in this
case, p is their momentum and h is Planck's constant. The electrons
are emitted from a heated filament in a cathode-ray tube and
accelerated through a constant voltage so they all have the same
momentum. The voltage is low enough that the non-relativistic
expression for kinetic energy may be used:
is the wave length of the particles, electrons in this
case, p is their momentum and h is Planck's constant. The electrons
are emitted from a heated filament in a cathode-ray tube and
accelerated through a constant voltage so they all have the same
momentum. The voltage is low enough that the non-relativistic
expression for kinetic energy may be used:
K.E. = eV = p²/(2m)
so
p = sqrt(2meV) (2)
and
= h/sqrt(2meV) (3)
Substituting numerical values for the physical constants in this equation, and expressing the result in nanometers gives
= 1.22643/sqrt(V) nm (V in volts) (4)
This numerical relation between the wavelength of the electrons and the voltage through which they have been accelerated is valid if and only if the DeBroglie relation (1) is valid. Thus to test the DeBroglie relation all we need is a means of measuring the electrons' wavelength. Clearly, according to Eq. (4), these wave- lengths are quite small. We need a diffraction grating with a spacing between lines of less than a nanometer. Such a "grating" is supplied by a typical crystal where the spacings between the atoms in the crystal are a few tenths of a nanometer.
In this experiment beams of electrons will be directed onto foils of aluminum and carbon. The beams have diameters large enough that in the case of aluminum millions of tiny crystals, randomly oriented, will be illuminated. Interference occurs between regularly spaced planes which contain large numbers of electrons. If the spacing between the planes is d then n-th order constructive inter- ference will occur if
n= 2d sin
(5)
where 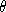 is the angle between the incident beam and the plane of the
crystal. The interference will be observed as a cone of apex half
angle equal to 2
is the angle between the incident beam and the plane of the
crystal. The interference will be observed as a cone of apex half
angle equal to 2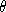 .
If at a distance R from the foil a circle of
radius r is observed, then
.
If at a distance R from the foil a circle of
radius r is observed, then
r/R = tan 2~ 2
~ 2 sin
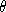
Thus
= (d/n) (r/R) (6)
In books on crystalography it is shown that the ratio d/n is given in terms of the lattice constant a, which is 0.4041 nm for aluminum, and three integers H, K and L, called the Miller indices, according to
d/n = a/sqrt(H² + K² + L²)
Thus the observed circles have radii such that
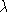 = a (r/R)/sqrt(H² + K² + L²) (8)
= a (r/R)/sqrt(H² + K² + L²) (8)
For a face-centered cubic lattice, such as aluminum, the integers H, K and L must be all odd or all even. Thus the allowed values are those listed below:
H K L H²+K²+L²
1 1 1 3
2 0 0 4
2 2 0 8
3 1 1 11
2 2 2 12
4 0 0 16
3 3 1 19
4 2 0 20
4 2 2 24
etc.
If instead of randomly oriented planes the electrons are diffracted from regular lattice sites as in a single crystal, one observes spots on the screen for definite directions of constructive interference. The crystals in the graphite foils are large enough for this to occur. The condition for constructive interference is again Eq. (4) but for a hexagonal crystal such as graphite one obtains instead of Eq. (8):
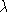 = a (r/R) sqrt(3)/2 (9)
= a (r/R) sqrt(3)/2 (9)
where the lattice constant a is 0.2456 nm for graphite, r is the distance between dots and R, as before, is the distance from the foil to the viewing screen.
Set the voltage and intensity controls to zero and then turn on the equipment. Allow a short time for warm up and then set the high voltage to 8000 volts. Slowly advance the intensity control until a bright spot is observed and use the focus control to reduce the size of the spot as much as possible. Then use the positioning controls to move the beam so that it strikes an aluminum foil, as indicated by the appearance of rings on the screen. The beam current should be between 5 and 10 µA. Measure the diameters of as many rings as possible. Repeat for different accelerating potentials. Suggested values are 5000 and 6500 volts. If necessary, somewhat higher beam currents may be used at the lower potentials.
By careful adjustment of the positioning controls, find a graphite foil which displays a regular hexagonal pattern of dots. Measure the distance between the center spot and the six nearest dots. Take any other measurements which will aid you in obtaining a good average value for the distance between dots. Repeat for three different accelerating voltages as described above.
For the aluminum foil data, determine the values of H, K and L
that correspond to each ring. For example, assume the smallest
ring has H² + K² + L² = 3. Compute values of the squares of
the ratios of the other rings to the smallest ring and compare
with the expected values of 4/3, 8/3, 11/3, 12/3, etc. If essential
agreement is obtained your assumption is probably correct and the
Miller indices correctly determined. Then calculate
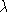 from Eq. (8)
for each ring obtained at a given potential, calculate the average
and compare it with
from Eq. (8)
for each ring obtained at a given potential, calculate the average
and compare it with 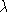 calculated from Eq. (4) for that potential.
Repeat for the other potentials. The value of R is given by the
manufacturer as 7.000 inches.
calculated from Eq. (4) for that potential.
Repeat for the other potentials. The value of R is given by the
manufacturer as 7.000 inches.
For the graphite data, proceed in essentially the same way with the
exception that Eq. (9) is used to calculate 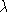 instead of Eq. (8).
instead of Eq. (8).
Eqs. (8) and (9) are true for any waves with the given
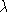 's while
Eq. (4) follows from the DeBroglie relation. Thus the success of
the above comparisons is direct proof of the validity of this
relation.
's while
Eq. (4) follows from the DeBroglie relation. Thus the success of
the above comparisons is direct proof of the validity of this
relation.
The data taken above permit a determination of Planck's constant,
based on the assumed validity of the DeBroglie relation. For each
potential, determine p from Eq. (2) and
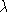 from Eq. (8) or (9) as
appropriate. The product should equal Planck's constant. What is
the average value of Planck's constant determined in this way?
from Eq. (8) or (9) as
appropriate. The product should equal Planck's constant. What is
the average value of Planck's constant determined in this way?
© Department of Physics and Astronomy, University of Iowa, Iowa City, Iowa. All rights reserved.